October 17th, 2025
The Zylka Lab enjoyed the nice weather and good company at UNC Employee Appreciation Day!
May 13th-17th, 2025
Carla Belmonte Mateos, Leo Blondel, and Luke James at the 2025 American Society of Gene and Cell Therapy Annual Meeting, held in New Orleans, May 13th through 17th. Carla was acknowledged for her Career Development Award, and Luke presented on his research in the Zylka and Philpot labs.
March 22nd & April 14th, 2025
Undergraduate Jenny Tran recently presented her research at the Chancellor's Science Scholars symposium and BIOL395 Research Symposium. Jenny has been conducting research on cytosine base editors under the mentorship of postdoc Carla Belmonte Mateos, PhD.
December 13th, 2024
Hannah Bazick successfully defended her PhD Thesis: “Towards an effective gene therapy for Angelman syndrome: Targeting UBE3A-ATS with CRISPR/Cas9 and zinc finger nucleases” on December 13th. During her time in the Zylka Lab, Hannah studied gene editing strategies with CRISPR/Cas9 and zinc finger nucleases. Her defense was celebrated by family and fellow lab members with a lunch after!
December 10th, 2024
Zylka Lab members dressed up for a holiday picture
December 2nd, 2024
Congratulations to postdoc Carla Belmonte Mateos, PhD, for receiving a Career Development Award from the American Society of Gene + Cell Therapy (ASGCT) for her research on CRISPR/Cas9-based genome editing strategies to investigate potential therapies for Angelman Syndrome. This award is intended to support independent transformative pilot studies in gene and cell therapy.
Carla Belmonte Mateos, PhD
October 31st, 2024
The Zylka Lab attended the Cell Biology & Physiology Halloween Party! Pictured is our submission for the pumpkin carving contest.
October 16th, 2024
Rami Major successfully defended her PhD Thesis: “Emergent Ethical and Scientific Considerations of Gene Editing in Human Disease” on October 16th. During her time in the Zylka Lab, Rami studied nuclear localization of Cas9 to improve CRISPR-Cas9 editing efficiency. Her defense was celebrated by family, friends, and fellow lab members with a goodbye reception!
May 16th, 2024
Ina Klockner, Hannah Bazick, Rami Major, and Luke James at the 2024 American Society of Gene and Cell Therapy Annual Meeting, held in Baltimore, May 7th through 11th.
July 27, 2023:
Luke James, Mark Zylka, Carla Belmonte-Mateos, Ina Klockner, and Hannah Bazik at the 2023 Angelman Syndrome Foundation Research Symposium, held in Nashville, July 25 and July 26.
.
June 8, 2023:
Several lab members enjoying time together at the 2023 Neuroscience Center spring retreat at Haw River State Park.
April 30, 2023:
Goodbye lunch at Top of the Hill for former postdoc Justin Wolter, who now has a faculty position and lab at the University of Wisconsin-Madison. (Sam Boeshore is joining Justin’s lab there, too.)
Left to right: Sam Boeshore, Wenxin Hu, Esther Park, Rami Major, Nik Morrison-Welch, Sophia Lamberti, Hannah Bazik, Lei Xing, Dan Ryan, Carla Belmonte-Mateos, Luke James, Ina Klockner, Justin Wolter, and Mark Zylka.
Wenxin Hu, Ph.D.
February 22, 2022:
Wenxin Hu, Ph.D. and co-workers have found measurable levels of a biomarker for azoxystrobin in pregnant women and young kids and investigated the fungicide’s ability to pass from mothers to embryos in utero in mice and during lactation. The resulting paper, “Detection of Azoxystrobin Fungicide and Metabolite Azoxystrobin-Acid in Pregnant Women and Children, Estimation of Daily Intake, and Evaluation of Placental and Lactational Transfer in Mice,” appears in Environmental Health Perspectives. More information here.
CHAPEL HILL, NC – For the first time, UNC-Chapel Hill researchers have measured the concentration of a biomarker of the commonly used fungicide azoxystrobin (AZ) in the urine of pregnant women and children ranging from 40-84 months of age. They also documented maternal transfer of AZ to mouse embryos and weaning-age mice.
The researchers’ experimental data, published in the journal Environmental Health Perspectives, also found that AZ entered the brain of mice in utero at concentrations that modeled environmentally relevant exposures. Using similar concentrations, the researchers then found that AZ killed some embryonic cortical neurons in cultures.
Jesse Niehaus, Ph.D. (right).
March 7, 2021:
A new cell type is implicated in neuropathic pain: “Spinal macrophages resolve nociceptive hypersensitivity after peripheral injury,” published in Neuron, lead author Jesse Niehaus, PhD. Details here.
CHAPEL HILL, NC – One of the hallmarks of chronic pain is inflammation, and scientists at the UNC School of Medicine have discovered that anti-inflammatory cells called MRC1+ macrophages are dysfunctional in an animal model of neuropathic pain. Returning these cells to their normal state could offer a route to treating debilitating pain caused by nerve damage or a malfunctioning nervous system.
The researchers, who published their work in Neuron, found that stimulating the expression of an anti-inflammatory protein called CD163 reduced signs of neuroinflammation in the spinal cord of mice with neuropathic pain.
Justin Wolter, Ph.D., and Hanqian Mao, Ph.D.
October 29, 2020:
The Zylka lab takes a step toward Angelman syndrome gene therapy with its publication “Cas9 gene therapy for Angelman syndrome traps Ube3a-ATS long non-coding RNA” published in Nature. The research, coordinated by Justin Wolter, Ph.D. and Hanqian Mao, Ph.D., demonstrates how gene editing with CRISPR-Cas9 can restore function in an animal model of the neurodevelopmental condition Angelman syndrome. More information here.
CHAPEL HILL, NC – October 21, 2020 – Babies born with a faulty maternal copy of the UBE3A gene will develop Angelman syndrome, a severe neurodevelopmental disorder with no cure and limited treatments. Now, for the first time, scientists at the UNC School of Medicine show that gene editing and gene therapy techniques can be used to restore UBE3A in human neuron cultures and treat deficits in an animal model of Angelman syndrome.
This work, published in Nature and led by senior author Mark Zylka, PhD, Director of the UNC Neuroscience Center and W.R. Kenan, Jr. Distinguished Professor of Cell Biology and Physiology, lays important groundwork for a long-lasting treatment or cure for this debilitating disease, as well as a therapeutic path forward for other single-gene disorders.
February 3, 2020: The Zylka lab was awarded a grant from the NINDS to develop a machine learning tool to detect and quantify mouse facial grimaces from videos. This tool can be used to evaluate the efficacy of drugs for chronic pain, among other applications.
September 18, 2019: The Zylka lab was awarded two grants from the NIH. One grant is focused on developing a CRISPR/Cas gene therapy for Angelman syndrome. The second grant is focused on studying autism-linked mutations in UBE3A. Details here.
June 5, 2019: The Zylka lab was awarded a $6.8M grant from the NIEHS to study environmental risks for autism, ADHD. Details here.
Goodbye dinner for Vicki
December 3, 2018: Vicki Brings defended PhD Thesis
Vicki Brings defended her PhD Thesis: “Characterization of diacylglycerol kinases eta and iota in itch, pain, and psychopathological behavior in mice” on November 7. Vicki has been a member of the Zylka Lab since 2013 and has spent her time in the lab studying pain signaling. Vicki’s successful defense was celebrated with a reception and goodbye dinner!
July 3, 2018: The Zylka Lab was awarded a grant from the Angelman Syndrome Foundation to expand their recent findings towards a gene therapy. Angelman Syndrome is a severe neurodevelopmental disorder that is caused by loss of the maternal copy of the Ube3a gene. Using the gene editing technology CRISPR/Cas9, a team of scientists in the Zylka lab was able to correct the underlying molecular deficiency in the Angelman mouse model. To read more, click here.
April 1, 2018: Mark Zylka receives Center for Environmental Health and Susceptability Pilot Award
The project, “Does prenatal pesticide exposure exacerbate phenotypes in a mouse model of autism?”, submitted for the 2018-2019 CEHS Standard Pilot Projects Program, was approved for funding on April 1, 2018.
Research for this pilot award explores the role of both genetic and environmental components of a single autism-related pathway in an effort to show more pronounced morphological and behavioral phenotypes than testing either genetic or environmental risk factors alone. This pilot grant will support development of assays to quantify pyrethroids in tissues and urine of mice and pilot exposure studies with a high confidence autism model mouse line.
November 20, 2017: Mark Zylka named American Association for the Advancement of Science Fellow
Mark J. Zylka, PhD, is the W.R. Kenan Distinguished Professor of Cell Biology and Physiology and director of the UNC Neuroscience Center. As part of the Section on Biological Sciences, Zylka was elected an AAAS Fellow for his distinguished contributions to the field of neuroscience, particularly for the study of autism-related genes and risk factors using high-throughput approaches. You can read more about his career here and about some of his latest work on potential environmental causes of autism here.
Election as an AAAS Fellow is an honor bestowed upon AAAS members by their peers. This year 396 members have been awarded this honor by AAAS because of their scientifically or socially distinguished efforts to advance science or its applications. This year’s AAAS Fellows will be formally announced in the AAAS News & Notes section of the journal Science on November 24, 2017. New Fellows will be presented with an official certificate during the 2018 AAAS Annual Meeting in Austin, Texas on February 17.
Mark Zylka, PhD
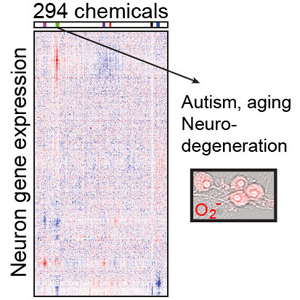
The study shows how a class of commonly used fungicides, designed to protect crops, can cause gene expression changes in mouse brain cells that look strikingly similar to changes in the brains of people with autism and Alzheimer’s disease. The work, published in the journal Nature Communications, describes a new way to home in on chemicals that have the potential to affect brain functions. Authors of the paper are Brandon Pearson, Jeremy Simon, Eric McCoy, Gabriela Salazar, Giulia Fragola, and Mark Zylka. (See also this piece in The Guardian.)
Jason Yi, Ph.D.
March 18, 2016: Zylka lab postdoc wins SFARI Bridge to Independence Award
Congratulations to Jason Yi for receiving a Bridge to Independence Award from the Simons Foundation Autism Research Initiative (SFARI) for his proposal, “Inhibitory circuit dysfunction in autism spectrum disorder.” These awards are activated upon starting a faculty position and are intended to invest in the next generation of top autism investigators by helping early-career scientists transition from mentored training positions to independent research careers. The award results in a commitment of $450,000 over three years.
Ben Philpot and Mark Zylka
January 14, 2016: Zylka and Philpot to lead UNC Neuroscience Center
Mark Zylka, PhD, will serve as director and Ben Philpot, PhD, will serve as associate director of the UNC Neuroscience Center at the UNC School of Medicine, effective July 1. William Snider, MD, who has served as the center’s director for nearly 17 years, will step down from his leadership role, but will remain on faculty as professor of neurology, while also continuing his research.
August 6, 2015: Zylka Lab Publishes in Cell on Genetic Mutation that Increases Autism Risk
In an article published in the journal Cell, Jason Yi and co-workers show how a genetic mutation disables a regulatory molecular switch, creating an enzyme that cannot be turned off and leading to abnormal brain development and autism. A video describing this work was aired on WRAL.
July 9, 2015: Mark Zylka Awarded RSRT Grant for Long Genes Screening
Mark Zylka and co-workers discovered that a class of drugs called topoisomerase inhibitors reduces the expression of long genes, raising the possibility that this class of drugs could be clinically relevant for Rett. The Rett Syndrome Research Trust has awarded Mark Zylka $400,000 to screen for other compounds of topoisomerase inhibitors that can rebalance expression of long genes safely.
Angela Mabb, Ph.D.
Post-doctoral fellow Angela Mabb and co-workers in the Philpot lab and the Zylka lab have discovered a biochemical mechanism that could be the cause of the neurological side effects of chemotherapy, including memory loss, confusion, difficulty thinking, and trouble concentrating. Their research, published in Proceedings of the National Academy of Sciences, shows how the drug topotecan can drastically suppress the expression of Topoisomerase-1, a gene that triggers the creation of proteins essential for normal brain functions. The authors also suggest that if these enzymes are affected during brain development, the result could be long-term neurodevelopmental problems, such as those found in people with Autism Spectrum Disorder.